The European Union has strengthened its chemical safety framework with Regulation (EU) 2024/2865, a key amendment to Regulation (EC) No 1272/2008 (the CLP Regulation). Published on 20 November 2024, this regulation ensures that the EU remains at the forefront of global chemical safety by addressing modern challenges like online sales, digital labelling, and emerging hazardous properties such as endocrine disruption and bioaccumulation.
The document describes Regulation (EU) 2024/2865 as a broader chemical safety regulation that applies to chemicals in general. It covers the classification, labelling, and packaging of chemical substances and mixtures across various industries, not just cosmetics. If you're specifically interested in cosmetics regulations, this appears to be a broader chemical safety regulation that would have implications for cosmetic product formulations and labelling. Please seek expert advice to get accurate information.
Here’s a comprehensive look at this regulation, why it matters, and how it impacts manufacturers, distributors, and importers.
A New Era for Chemical Classification, Labelling, and Packaging
The original CLP Regulation, implemented in 2009, was built on the globally recognised GHS framework (Globally Harmonised System of Classification and Labelling of Chemicals). This legislation established consistent rules for classifying hazards, labelling products, and packaging chemicals for safe handling. However, as technology and markets evolved, the need for a more dynamic approach became evident.
With this amendment, the EU introduces vital updates that:
Add new hazard classes to address risks like endocrine disruption and persistent, mobile, and toxic substances (PMT).
Improve hazard communication by requiring digital labels for better accessibility and usability.
Simplify packaging standards, including provisions for fold-out labels on space-constrained products.
Enhance safety and sustainability through specific rules for refillable chemical products.
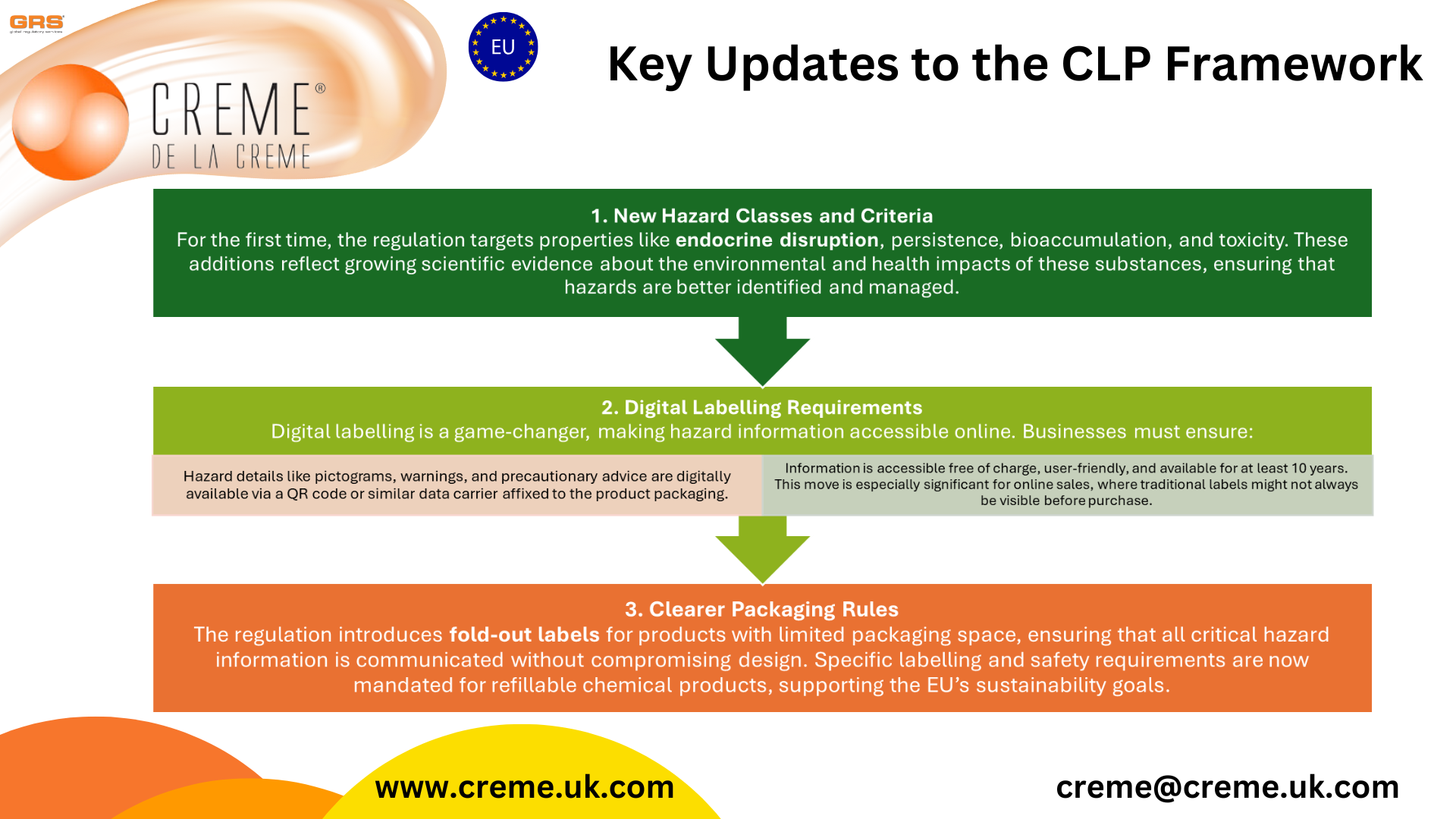
Key Updates to the CLP Framework
New Hazard Classes and Criteria
For the first time, the regulation targets properties like endocrine disruption, persistence, bioaccumulation, and toxicity. These additions reflect growing scientific evidence about the environmental and health impacts of these substances, ensuring that hazards are better identified and managed.Digital Labelling Requirements
Digital labelling is a game-changer, making hazard information accessible online. Businesses must ensure:Hazard details like pictograms, warnings, and precautionary advice are digitally available via a QR code or similar data carrier affixed to the product packaging.
Information is accessible free of charge, user-friendly, and available for at least 10 years.
This move is especially significant for online sales, where traditional labels might not always be visible before purchase.
Clearer Packaging Rules
The regulation introduces fold-out labels for products with limited packaging space, ensuring that all critical hazard information is communicated without compromising design. Specific labelling and safety requirements are now mandated for refillable chemical products, supporting the EU’s sustainability goals.
Impact on Stakeholders
For Manufacturers
Manufacturers must reassess their products to ensure compliance with the updated classification criteria, particularly for the new hazard classes. This involves updating physical and digital labels while integrating fold-out designs, QR codes, or other linear barcodes where necessary. Adapting to the latest safety standards is critical for companies producing refillable products.For Distributors
Distributors play a vital role in maintaining the supply chain’s compliance. This includes verifying that all products carry the required physical and digital labels and ensuring that imported goods meet EU standards.For Importers
Importers must confirm that chemicals brought into the EU adhere to the updated classification, labelling, and packaging requirements. Collaboration with non-EU suppliers will be essential to meeting the regulation’s stringent demands.
Transition Timelines
The regulation provides specific deadlines to ensure businesses have time to comply:
By 20 May 2025 (6 months after entry into force): Products requiring new or more severe hazard classifications must comply.
By 20 May 2026 (18 months after entry into force): Products with less severe changes or downgraded classifications must comply.
These timelines ensure a phased rollout, minimising disruption while encouraging proactive compliance.
FAQs: What You Need to Know
What is the purpose of Regulation (EU) 2024/2865?
It modernises the EU’s chemical safety framework by introducing new hazard classes, digital labelling requirements, and improved packaging standards to protect health and the environment.What is digital labelling, and how does it work?
Digital labelling requires businesses to provide hazard information online via a data carrier like a QR code or other linear bar codes. This ensures consumers can access safety details easily and in multiple languages.How do the new hazard classes affect my products?
If your products fall under the new hazard classes (e.g., endocrine disruptors or PMT substances), you must reclassify, relabel, and repackage them by the required deadlines.Are there any changes for refillable products?
Yes, the regulation introduces specific rules to ensure that refillable products meet safety and labelling requirements, aligning with the EU’s sustainability goals.What happens to products already on the market?
Products placed on the market before the compliance deadlines are exempt from relabelling, providing some relief to businesses managing existing stock.
Conclusion
Regulation (EU) 2024/2865 represents a forward-thinking approach to chemical safety. Incorporating digital tools, addressing emerging hazards, and promoting sustainability strengthens the EU’s commitment to protecting people and the planet. Whether you’re a manufacturer, distributor, or importer, staying ahead of these changes is essential for compliance and competitiveness in the EU market.
For more details, consult the regulation's official text or seek guidance from regulatory experts to ensure your business is ready for this new chapter in chemical safety.
Let Crème de la Crème be your trusted partner in fully complying with the EU’s evolving regulatory framework. Whether you’re a small business or a multinational corporation, we’re here to ensure your success.
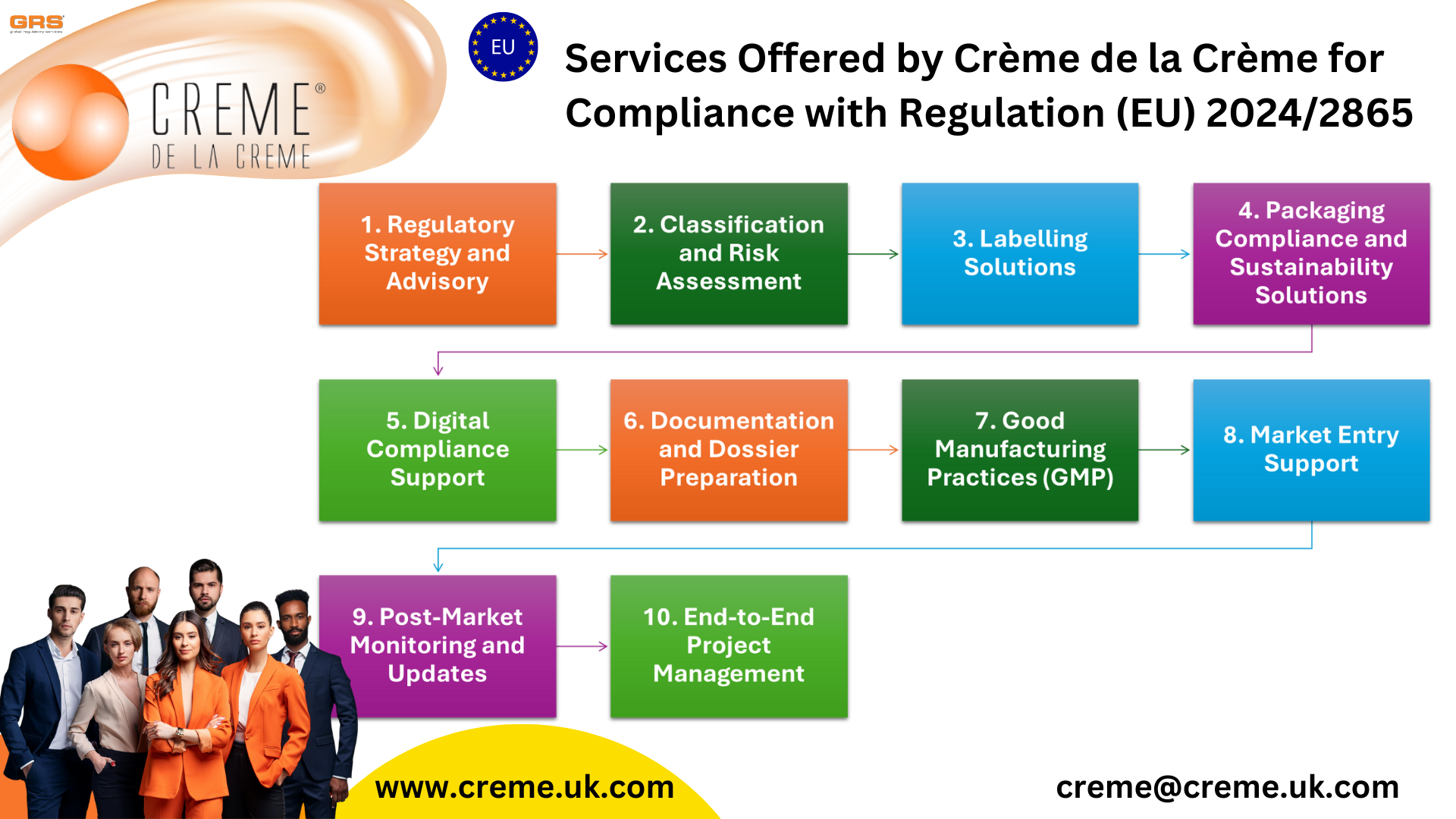
Services Offered by Crème de la Crème for Compliance with Regulation (EU) 2024/2865
Crème de la Crème positions itself as a one-stop regulatory consultancy, providing end-to-end solutions to help clients comply with Regulation (EU) 2024/2865. Our tailored services span from classification and labelling to packaging and ongoing compliance monitoring, ensuring that businesses of all sizes can meet the stringent requirements of the regulation with ease and efficiency.
Regulatory Strategy and Advisory
Gap Analysis: Assess your current compliance status against the requirements of Regulation (EU) 2024/2865, identifying areas that need updating.
Compliance Roadmaps: Develop customised strategies to align your processes, products, and systems with the updated regulation.
Regulatory Intelligence: Provide continuous updates on changes in EU regulations, including new hazard classes, digital labelling, and packaging requirements.
Classification and Risk Assessment
Substance and Mixture Classification: Conduct hazard classification for substances and mixtures based on the updated criteria, including the newly introduced hazard classes (e.g., endocrine disruptors, PBT, and PMT substances).
Risk Assessments: Evaluate the potential risks associated with your products to ensure accurate classification and labelling.
Scientific Justification: Provide robust documentation and justification for classifications to meet regulatory scrutiny.
Labelling Solutions
Create and integrate QR codes [Barcodes]or other data carriers linking to hazard information.
Ensure that digital labels are compliant with accessibility requirements, including multi-language support and usability for vulnerable groups.
Physical Labelling Design:
Assist in designing traditional and fold-out labels for products with limited space.
Ensure compliance with all labelling requirements, including hazard pictograms, signal words, hazard statements, and precautionary advice.
Advertising Compliance: Ensure that online product advertisements meet the clarified labelling requirements for digital and distance sales.
</ul></li>Packaging Compliance and Sustainability Solutions
Packaging Design Advisory: Develop packaging solutions that balance regulatory compliance with sustainability goals.
Refillable Product Solutions: Provide specific labelling and safety guidelines for refillable chemical products, supporting compliance and environmental goals.
Fold-out Labels: Design fold-out labels to accommodate all required information for products with limited space.
Digital Compliance Support
Ensure that products sold through e-commerce platforms display hazard information clearly and in compliance with the regulation.
Work with online retailers to integrate digital labelling at the point of sale.
Data Management: Set up systems to maintain digital hazard information for at least 10 years, as required by the regulation.
</ul></li>Documentation and Dossier Preparation
Technical Documentation: Prepare and maintain detailed records, including classification justifications, labelling data, and packaging specifications.
Submission Dossiers: Create regulatory dossiers for harmonised classification proposals and notifications to the European Chemicals Agency (ECHA).
Product Safety Reports: Compile Cosmetic Product Safety Reports (CPSR) for relevant products.
Good Manufacturing Practices (GMP)
GMP Implementation: Assist businesses in implementing ISO 22716 and other GMP standards to ensure safe and compliant manufacturing processes.
Audit Support: Conduct internal audits to verify compliance with regulatory standards.
Market Entry Support
Pre-Market Approvals: Ensure that new products meet all regulatory requirements before market entry.
Multi-Market Compliance: Adapt classification, labelling, and packaging for compliance across multiple jurisdictions, including the EU, UK, USA, and India.
Representation Services: Act as an EU or UK Responsible Person (RP) for regulatory compliance.
Post-Market Monitoring and Updates
Ongoing Compliance Monitoring: Track regulatory changes and their implications for your products, ensuring continued compliance.
Incident Management: Provide support in responding to safety incidents or regulatory inspections.
Voluntary Updates: Help you adopt new regulatory measures proactively before mandatory deadlines to maintain a competitive edge.
End-to-End Project Management
Handle all aspects of compliance, from initial classification and labelling to post-market monitoring, as a turnkey solution.
Act as a single point of contact for all regulatory matters, streamlining communication and ensuring efficiency.
Digital Labelling Implementation:
Online Sales Compliance:
Why Choose Crème de la Crème?
With our deep expertise in regulatory compliance and commitment to client success, we simplify the complex requirements of Regulation (EU) 2024/2865. By partnering with us, you gain access to:
Personalised support tailored to your business needs.
Expertise in handling the nuances of both local and international regulations.
Innovative solutions to balance compliance, sustainability, and market competitiveness.
Let Crème de la Crème be your trusted partner in achieving full compliance with the EU’s evolving regulatory framework. Whether you’re a small business or a multinational corporation, we’re here to ensure your success. Contact us today to discuss how we can help!